selenium orbital diagram|selenium electron configuration : Tagatay Atoms can jump from one orbital to another orbital in an excited state. This is called quantum jump. The ground state electron configuration of selenium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p4. In the selenium . Tingnan ang higit pa To make the app more interesting, Android users can choose to customize their experiences with WhatsApp Messenger, using the provided mod. Simply download the WhatsApp Messenger APK on our .
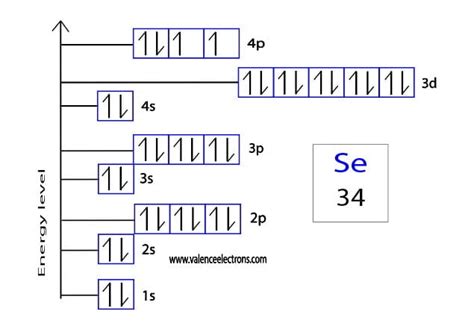
selenium orbital diagram,Atomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable region of electron rotation around the nucleus is called the orbital. The sub-energy levels depend on the azimuthal quantum number. It is expressed by ‘l’. The . Tingnan ang higit pa
The total number of electrons in selenium is thirty-four. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons . Tingnan ang higit paScientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the . Tingnan ang higit paThe electron configuration of selenium shows that the last shell of selenium has six electrons. Therefore, the valence electrons of seleniumare six. The elements that have . Tingnan ang higit paAtoms can jump from one orbital to another orbital in an excited state. This is called quantum jump. The ground state electron configuration of selenium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p4. In the selenium . Tingnan ang higit pa To write the orbital diagram for the Selenium atom (Se) first we need to write the electron configuration for just Se. To do that we need to find the number of electrons . The selenium orbital diagram is a graphical representation of the electron configuration of the selenium atom. This diagram shows how the electrons in the .
This video shows how to draw the orbital diagram of selenium (Se). It also shows how to write the electron configuration of selenium (Se) and the shorthand noble . Steps. Here’s how you can draw the orbital diagram of selenium step by step. #1 Find electrons of selenium. #2 Write electron configuration of selenium. #3 Draw . A step-by-step description of how to write the electron configuration for Selenium (Se) and the Selenium ion (Se2-).In order to write the Se electron configu.
We can write the electron configuration of selenium using four different methods: #1 Using aufbau principle. #2 Using periodic table. #3 From its Bohr model. #4 From its orbital diagram. Let’s break down . Selenium has 6 electrons in its outermost shell i.e. 2 electrons in s orbit and 4 electrons in the p orbit. Hydrogen Valence Electrons. Helium Valence Electrons. Lithium Valence Electrons. .The orbital diagram for selenium can be represented by using boxes to symbolize the orbitals and arrows to represent the electrons. Each box represents an orbital, and the .

Selenium electron configuration diagram. The method of distribution of the electrons in the orbitals of the Se atom is followed by the 2 important points are. The . The method of entering into the orbitals is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 4, the electronic configuration of the selenium atom. Selenium electron configuration diagram. The method of distribution of the electrons in the orbitals of the Se atom is followed by the 2 important points are. The orbital, which has minimum energy has to fill .selenium orbital diagram selenium electron configuration To write the orbital diagram for the Selenium atom (Se) first we need to write the electron configuration for just Se. To do that we need to find the number .
Electronic Configuration: The general representation of electrons in subshells, orbitals, shells has been done with the help of electronic configuration. Some of the different ways are: Condensed electronic configuration. Complete electronic configuration. Orbital electronic configuration.The orbital diagram is a visual representation of the electron configuration of an atom. It shows the arrangement of electrons in different energy levels or orbitals. In the case of selenium, the orbital diagram helps us understand how its 34 electrons are distributed among the energy levels. In selenium, the atomic number 34 indicates that it .Using an orbital diagram, determine the number of unpaired electrons in gallium. Write orbital diagrams for each ion and indicate whether the ion is diamagnetic or paramagnetic. a. C d 2 + b. A u + c. M o 3 + d. Z r 2 + Write orbital diagrams for each ion and indicate whether the ion is diamagnetic or paramagnetic. a. V^5+ b. Cr^3+ C. Ni^2+ d .Orbital Diagrams. An orbital diagram, like those shown above, is a visual way to reconstruct the electron configuration by showing each of the separate orbitals and the spins on the electrons. This is done by first determining the subshell (s,p,d, or f) then drawing in each electron according to the stated rules above.
selenium electron configurationTo write the orbital diagram of copper, you have to write the orbital notation of copper. Which has been discussed in detail above. Copper orbital diagram. 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electrons will first enter the 1s orbital. A step-by-step description of how to write the electron configuration for Selenium (Se) and the Selenium ion (Se2-).In order to write the Se electron configu.

Osmium orbital diagram. 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electrons will first enter the 1s orbital. According to Hund’s principle, the first electron will enter 1s orbital in the clockwise direction and the next electron will enter the 1s orbital in the anti-clockwise direction.
To draw the selenium Bohr model, outline the 34 protons, 45 neutrons, and 34 electrons. Start by illustrating the nucleus, and then draw the four electron shells. The first three shells should contain 2, 8, and 18 electrons, respectively, while the fourth shell holds the remaining 6 electrons. Steps. Write protons, neutrons, and electrons of . Lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol. Lewis electron dot diagrams for ions have less (for cations) or more (for anions) dots than the corresponding atom. This page titled 9.2: Lewis Electron Dot Diagrams is shared under a CC BY-NC-SA 3.0 license and was authored, remixed, .
To write the orbital diagram of chlorine, you have to write the orbital notation of chlorine. Which has been discussed in detail above. Chlorine (Cl) orbital diagram. 1s is the closest and lowest energy orbital to the nucleus. Therefore, the .
The lowest energy orbitals are closest to the nucleus and the higher energy orbitals are progressively further away from the nucleus in order of their energy levels. To write the orbital diagram of indium, you have to write the orbital notation of indium. Which has been discussed in detail above. Indium orbital diagram.For each configuration, (1) indicate the core electrons, (2) the outer electrons, and (3) draw the electron orbital diagram for the outer electrons. (a) Sc (b) Mn (c) Cd (d) Fe. Write the condensed electron configuration, the noble gas abbreviation, and draw the orbital diagram for the following element. (Remember to apply Hund's Rule, Pauli's .
Selenium atoms have 34 electrons and the shell structure is The ground state electron configuration of ground state gaseous neutral selenium is Ar. There are no d-orbitals in selenium which contributes to its nonreactivity. , Heres how you can draw the orbital diagram of selenium step by step. Step 1 find electrons of selenium Step 2 write .Draw the abbreviated orbital diagram for selenium (Se). How many valence electrons are in an atom of selenium? Expert Solution. This question has been solved! Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts. This is a popular solution! SEE SOLUTION Check out a sample Q&A here.selenium orbital diagramThe boxes are arranged in order of energy of the orbitals. The lowest energy orbitals are closest to the nucleus and the higher energy orbitals are progressively further away from the nucleus in order of their energy levels. 1. Orbital Diagram for Hydrogen (H) Hydrogen orbital diagram. 2.
selenium orbital diagram|selenium electron configuration
PH0 · what does orbital mean
PH1 · selenium electron configuration
PH2 · orbital diagrams chemistry worksheet
PH3 · orbital diagram calculator
PH4 · noble gas configuration for selenium
PH5 · how to draw molecular orbital diagrams
PH6 · Iba pa